The project “dealii-X: an Exascale Framework for Digital Twins of the Human Body” is part of the EuroHPC Centres of Excellence, with a primary goal of developing a scalable computational platform to generate accurate digital replicas of human organs. Leveraging the deal.II library, the project focuses on creating finite element models to approximate solutions for partial differential equations, a technique commonly used in engineering applications. The project aims to apply this methodology to biomechanics, specifically in modeling organs such as the lungs, heart, brain, liver, and cell mobility processes.
In the realm of biomedicine, computational models have traditionally been limited in their scope and accuracy. However, advancements in computing power and mathematical modeling have paved the way for more detailed and realistic organ models. These digital twins provide crucial insights into medical conditions and potential treatment strategies, shedding light on the underlying mechanisms driving various diseases.
The development of accurate organ models presents unique mathematical challenges, as each individual’s organ configuration is distinct and undergoes continuous changes throughout their lifetime. To create precise models, the dealii-X project must consider a wide range of scales, from cellular interactions to tissue-level mechanics involving complex materials like hyperelastic substances. By harnessing exascale computing capabilities, the project endeavors to simulate these intricate biological processes at an unprecedented level of detail, with initial results beginning to emerge since its inception in October 2024.
Respiratory mechanics
One critical aspect of the project focuses on respiratory mechanics, particularly in understanding the effects of mechanical ventilation on lung function. Computational models offer a way to predict the impact of different ventilation strategies on patient-specific lung geometries, addressing challenges that are difficult to observe through traditional medical imaging techniques.
Current models often overlook essential factors in respiratory mechanics, such as the role of surfactants in lung function. By integrating surfactant dynamics into patient-specific models, researchers at the Technical University of Munich, Germany, led by Professor Wolfgang A. Wall and Buğrahan Temür, utilize the ExaDG software project to conduct highly resolved simulations of alveolar structures. This software, based on the deal.II library, enables efficient solvers for fluid mechanics and solid mechanics problems, enhancing the understanding of surfactant effects on lung compliance.
Matrix-free cardiovascular simulations
Another critical area of focus within dealii-X is cardiovascular simulations, essential for studying heart function and blood circulation dynamics. High-fidelity 3D cardiac models require significant computational resources due to the complex interplay between blood flow, tissue mechanics, and biochemical processes. To address this challenge, researchers at Politecnico di Milano, Italy, led by Professor Luca Dede’ and his team, introduce a matrix-free computational framework for large-scale cardiovascular simulations. This approach, as shown in Fig. 2, computes operations on the fly, reducing memory usage and enhancing computational efficiency.
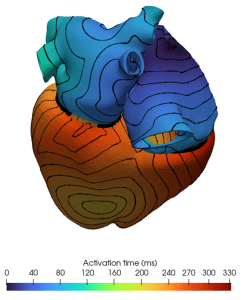
By exploring the impact of matrix-free algorithms on cardiovascular simulations, the team at Politecnico di Milano aims to enhance computational efficiency and resource utilization, paving the way for more personalized and data-driven approaches to cardiovascular care.
Gaining new insights into the human brain by inverse modelling of tissue mechanics
Advancements in understanding the biomechanical properties of human brain tissue can significantly improve the diagnosis and treatment of brain-related conditions. Through inverse modeling approaches, researchers can calibrate material model parameters for patient-specific brain models, aiding in preoperative planning and surgical interventions with minimal invasiveness. The ExaBrain project, developed by Professor Silvia Budday and Alexander Greiner at Friedrich-Alexander-University of Erlangen-Nuremberg in Germany, leverages high-performance computing methods to enable both parameter identification and full brain simulations, utilizing the deal.II library for accuracy and efficiency.
At the foundation of human cells
Cell motility plays a crucial role in processes like tumour metastasis and embryogenesis, driven by intricate mechanisms involving actin polymerization and cell membrane interactions. To enhance the understanding of cellular dynamics, researchers at the Università degli Studi di Brescia, Italy, led by Professor Alberto Salvadori, develop novel continuum multiphysics equations to govern cellular motility. By integrating these models into the deal.II suite, the team aims to advance mechanobiological research and develop therapies to regulate cell behavior and mobility.
Summary
The dealii-X project showcases a diverse range of models and developments, spanning from cardiac simulations to brain tissue mechanics. By harnessing the power of large-scale computing, researchers can explore new frontiers in biomedicine and beyond, driving innovation in mathematical modeling and computational science. The collaborative nature of the project fosters interdisciplinary research, offering insights that extend beyond the realm of healthcare into various scientific disciplines.
References
- https://dealii.org
- S. M. K. Rausch, D. Haberthür, M. Stampanoni, J. C. Schittny, and W. A. Wall. “Local Strain Distribution in Real Three-Dimensional Alveolar Geometries”. In: Annals of Biomedical Engineering 39.11 (2011), pp. 2835–2843.
- L. Wiechert, R. Metzke, and W. A. Wall. “Modeling the Mechanical Behavior of Lung Tissue at the Microlevel”. In: Journal of Engineering Mechanics 135.5 (2009), pp. 434–438.
- R. Schussnig, N. Fehn, P. Munch and M. Kronbichler. “Matrix-Free Higher-Order Finite Element Methods for Hyperelasticity”. In: Computer Methods in Applied Mechanics and Engineering 435 (2025), p. 117600.
- M. Fedele, R. Piersanti, F. Regazzoni, M. Salvador, P. C. Africa, M. Bucelli, et al. and A. Quarteroni. “A comprehensive and biophysically detailed computational model of the whole human heart electromechanics”. In: Computer Methods in Applied Mechanics and Engineering 410 (2023), p. 115983.
- M. Bucelli. “The lifex library version 2.0”. In: ACM Transactions on Mathematical Software (2024).
- P. C. Africa, M. Salvador, P. Gervasio, L. Dede, and A. Quarteroni. “A matrix–free high–order solver for the numerical solution of cardiac electrophysiology”. In: Journal of Computational Physics 478 (2023), p. 111984.
- M. Serpelloni, M. Arricca, C. Ravelli, E. Grillo, S. Mitola, A. Salvadori (2023), Mechanobiology of the relocation of proteins in advecting cells: modeling, experiments, and simulations, Biomechanics and Modeling in Mechanobiology, 22:1267–1287
- A. Salvadori, C. Bonanno, M. Serpelloni, R.M. McMeeking, (2024), On the generation of force required for actin-based motility., Scientific Reports, 14:18384
Authors
Martin Kronbichler (coordinator)
Silvia Budday
Luca Dede’
Alberto Salvadori
Wolfgang A. Wall